Optical spectroscopy
The method of ultraviolet circular dichroism (CD) is used in our group to gain information on secondary and tertiary structure, as well as on conformational changes of membrane-active peptides and proteins on a global scale. CD is based on differential absorption of left- and right-handed circularly polarized light due to chirality centers nearby to the peptide bonds and other chromophores contained in these biopolymers. This rather fast and sensitive technique is particularly useful for measuring biomacromolecules in the solution state but it can be also applied to membrane-bound proteins reconstituted in micellar solutions or vesicular suspensions. By applying state-of-the-art secondary structure evaluation algorithms for the deconvolution of the collected CD spectra we can quantify the fractions of the different secondary structure elements in the peptide systems under investigation. CD is also a valuable technique to detect conformational changes caused by a variation in the peptide sequence, e.g., by an amino acid mutation, due to changes in the protein/peptide environment (pH, temperature, concentration, solvents, liposomes, denaturant) or due to interactions with ligands, other proteins or nucleic acids.
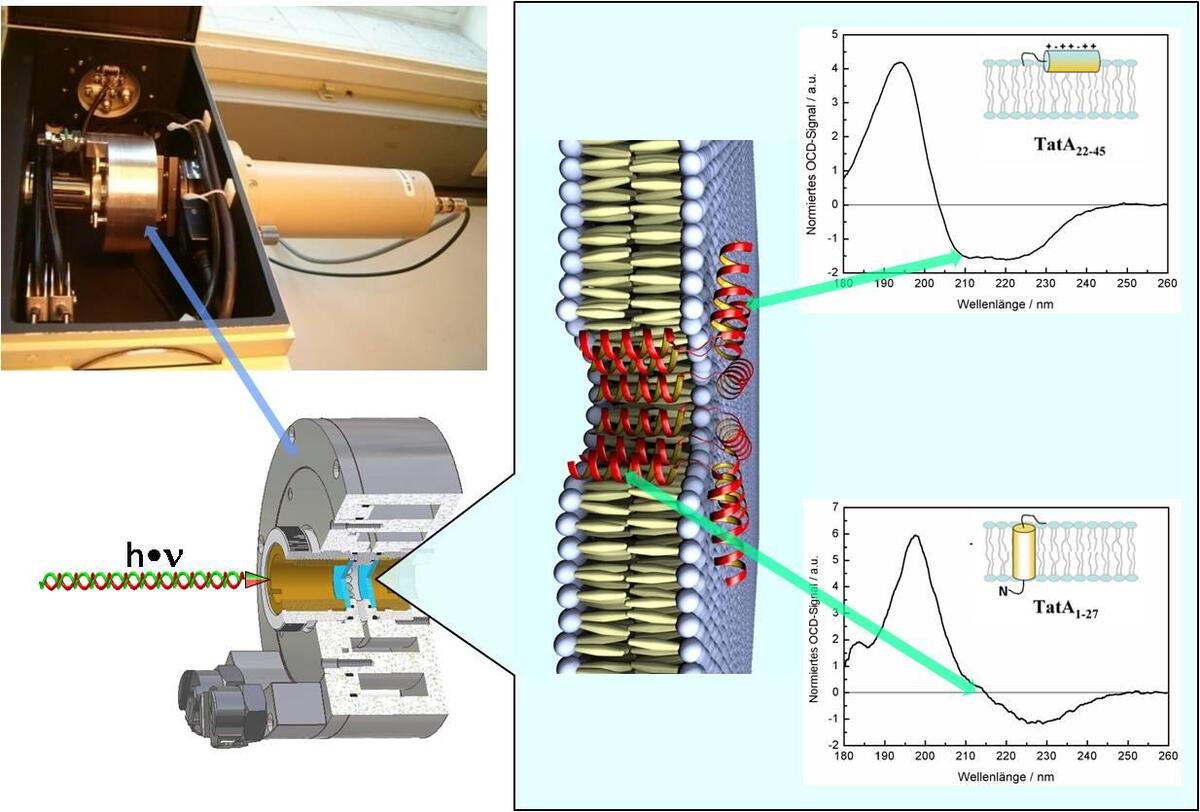
Our current NMR approach using single labels on synthetic peptides in oriented samples still suffers from the work- and cost-intensive steps necessary to prepare and analyze a series of multiple related peptides analogues. Therefore, we have implemented oriented circular dichroism (OCD) as a complementary spectroscopic technique which can reveal the qualitative alignment and conformation of a peptide in the membrane with high sensitivity and without the need for isotope labelling. We have built a rotatable sample holder for oriented membranes at controlled temperature and humidity, which can be integrated in a commercial spectropolarimeter. The spectra of antimicrobial (PGLa, MSI-103, Magainin) and cell-penetrating peptides (MAP, Transportan, etc.) under investigation are promising and clearly reveal the same re-alignment that has been observed by solid state NMR under comparable conditions. With this new OCD method at hand we are thus in a position to speed up the process of screening the experimental conditions under which interesting and functionally relevant structural changes occur in our peptides. After identifying the key conditions by OCD, such peptide-lipid systems can then be specifically studied by solid state NMR with quasi-atomic resolution. By applying OCD we also have verified the alignment and global fold of integral membrane proteins in oriented bilayers, e.g., TatA, various TatA constructs and TatCd, which are part of the Tat translocase system, as well as of the transmembrane sections of the oncoprotein E5 and its target, the PDGF-β receptor.
Synchrotron radiation-based CD (SRCD) in the vacuum-ultraviolet spectral range offers a number of advantages compared to conventional bench top instruments, the most important being the extension of the lower wavelength limit well below 190 nm and the considerably improved signal-to-noise ratio. Access to this extended spectral range allows a far more powerful identification of protein fold motifs. It is possible to discriminate 7-9 distinct motifs (such as different types of helices, parallel and antiparallel β-sheets, β-turns, polyproline type II folds, and disordered structures) compared with the 3-4 types of structures that can be resolved by conventional CD. In collaboration with ANKA we are currently setting-up a high-performance SRCD station by integrating the CD12 beamline (http://ankaweb.fzk.de/website.php?page=instrumentation_beam&id=21&field=1) from SRS Daresbury, U.K., which was transferred to Karlsruhe after the shutdown of the Daresbury synchrotron. Besides the usual SRCD experimental set-up, we will add our OCD accessory thus creating the world´s first SR-OCD beamline. A microfluidic stopped-flow set-up will complement the experimental station to monitor protein / peptide folding and fast conformational changes by SRCD and simultaneous FTIR measurements.
DICHROWEB (On-line deconvolution for protein circular dichroism spectra)
http://dichroweb.cryst.bbk.ac.uk/html/home.shtml
PCDDB (Protein Circular Dichroism Data Bank)
http://pcddb.cryst.bbk.ac.uk/home.php
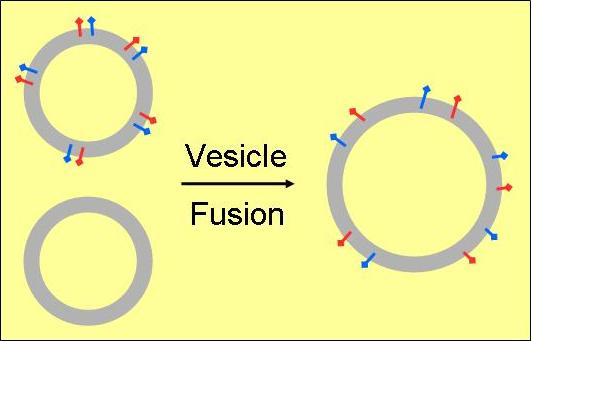
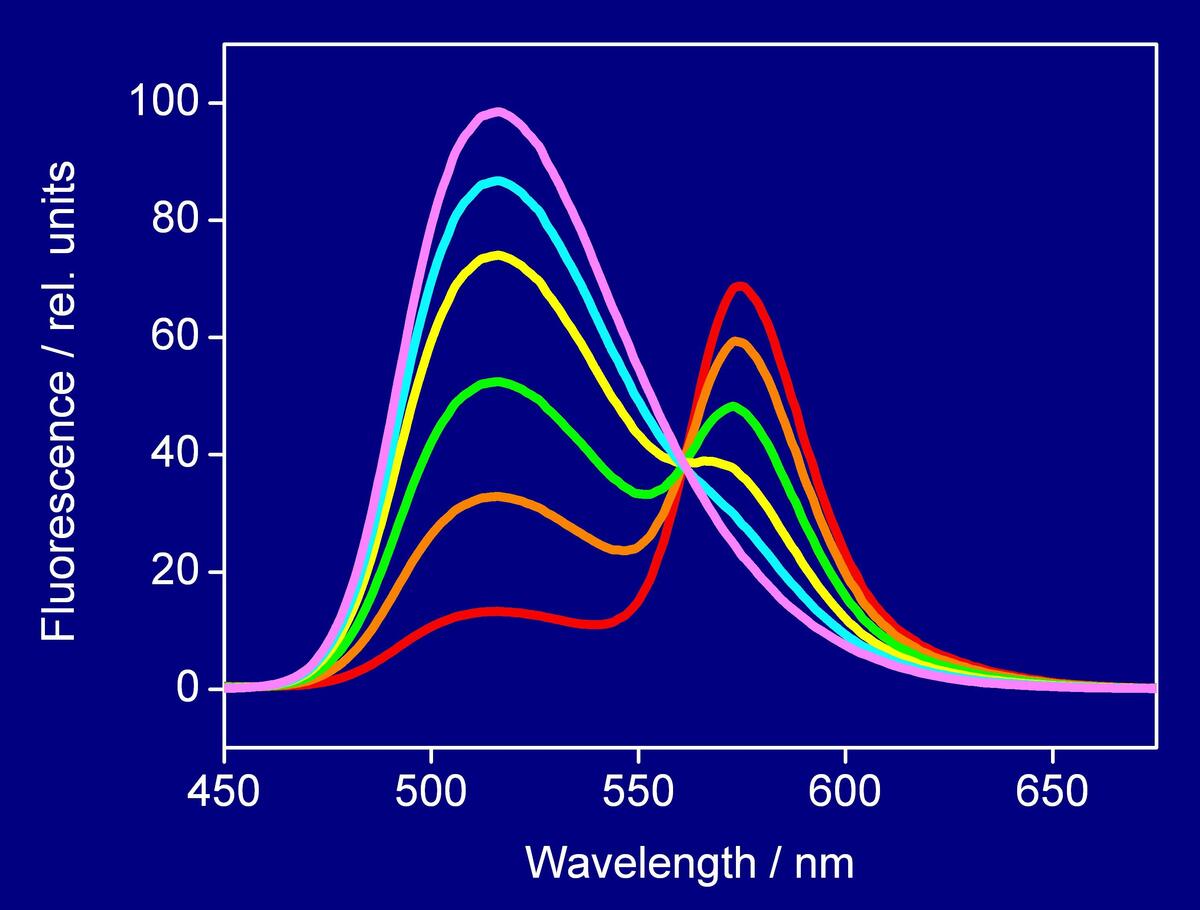
Additional optical spectroscopic techniques, such as fluorescence-, UV/VIS-spectroscopy, and light-scattering are applied to supplement structural investigations on peptides and proteins while interacting with membranes. To monitor the different functionalities (antimicrobial, cell-penetrating, or fusogenic) various assays based on lipid vesicles are used by which their specific action can be simulated in vitro.
In line with these investigations the underlying mechanisms can be elucidated yielding to a better understanding of the structural requirements by which most active peptide sequences are characterised. In this context the analyses are focused on fusogenic peptides as vesicle fusion represents a key role in many biological processes, and on cell-penetrating sequences in view of development of most efficient cargo delivery systems.