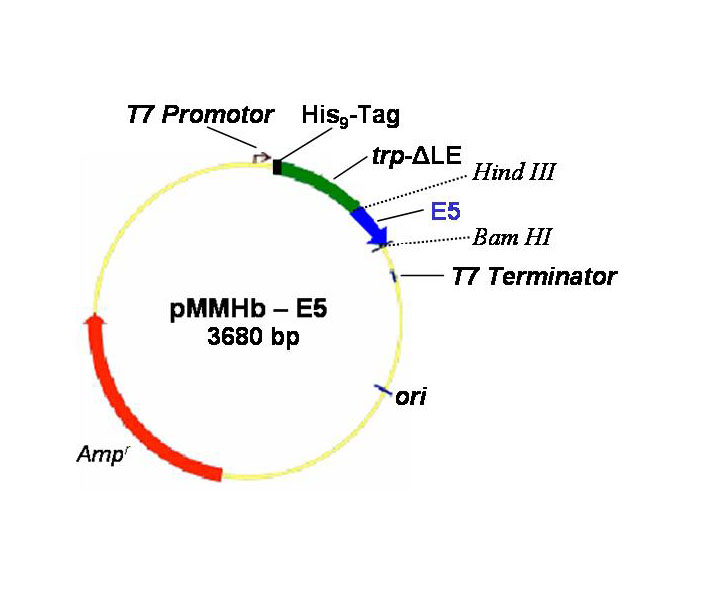
Bio Tools
The main assignment of BioLab is to produce labelled membrane proteins and membrane-active peptides of our interest for NMR-analysis as well as to carry out bioassays for characterisation of antimicrobial and haemolytic activity of chemically synthesized by peptide synthesis 3D-, 15N- and 19F-labelled peptide derivatives. How peptide penetrate and alter the cell membrane we observe using fluorescence microscopy and electron microscopy in collaboration with LEM, KIT.
Gramicidin S, which is selective against Gram-positive bacteria and Polymyxin B, which is selective against Gram-negative bacteria are in our BioLab a standard, model peptides. These peptide allow us to be sure in results by checking antimicrobial activity of chemically synthesized labelled peptides in follows methods:
Due to antimicrobial peptides are not only able to destabilize bacterial membrane, but also membrane of red blood cells, we characterize their activity against this cells in haemolytic assay with human erythrocytes. Gramicidin S shows a high haemolytic activity (sample movie) and we use this peptide in experiments as a positive control. Haemoglobin release and appearance of erythrocyte ghost after 2 min exposure with 20 µg Gramicidin S/ml could be observed live in Axioscop 40 (Carl Zeiss) under phase contrast with Objectiv “A-Plan” 100x / oil Ph3. For determination of minimal haemolytic concentration, for example, 50% haemolytic activity of peptides (HC50), we measure haemoglobin release of final erythrocyte concentration 0.5% at 420 nm (red points) or at 540 nm (blue points) in comparison to entirely haemolysis (HC100), which we obtain by addition of 0.1% Triton X-100. We plan also to study cytotoxicity of antimicrobial and cell penetrating peptides on some human cell cultures.